- Products & Solutions
- Events & Resources
- Support
- Customers
- Company
- Products & Solutions
- Events & Resources
- Support
- Customers
- Company
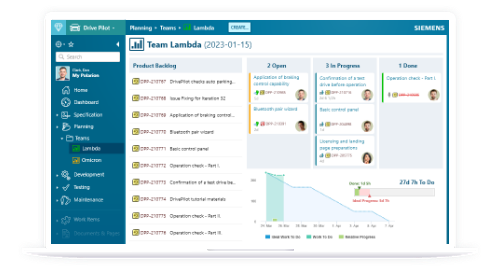
Everything you need to achieve agility and have full control over your cyber-physical systems application lifecycle.

Using Polarion ALM has provided us with a good user experience and solved the low adoption problem in our R&D management platform.

In terms of Polarion’s functionality and capabilities, there was no question. This presented a significant advantage.

Because we have everything in Polarion, everything is quite easily documented.

If we didn't have Polarion it would be very difficult to track everything that the car has to do.